Efavirenz is an important drug for treating HIV infection, but it has negative effects that can significantly impact patients’ quality of life over time. It causes neuropsychiatric disorders and neurocognitive impairment in roughly 50 percent of patients. The drug is associated with abnormal lipid levels in blood plasma, but the molecular mechanisms responsible for negative clinical observations are unknown.
 Nav Raj Phulara (courtesy of Phulara)
Nav Raj Phulara (courtesy of Phulara)
A new study in ACS Pharmacology & Translational Science led by chemistry Ph.D. student Nav Raj Phulara, used a novel combination approach to increase understanding of the relevant mechanisms. First, tissue imaging showed that Efavirenz alters lipid metabolism in mouse brains. Next, the researchers investigated all of the proteins present in the mouse brain sections and found that Efavirenz downregulates certain enzymes. All of these changes could be responsible for the drug’s negative neuropsychiatric effects. If proven so, new drugs could potentially be developed to block the negative activity of Efavirenz while allowing its positive effects to continue.
“Lipid abnormalities in the brain can lead to adverse effects like brain disorders and neurodegenerative diseases, and the brain is rich in lipids overall,” says Herana Kamal Seneviratne, assistant professor of chemistry and biochemistry and senior author on the new paper. “That formed the basis for investigating lipids in this study.”
The new combination approach could also be applied to investigate lipid metabolism in other systems, such as the heart and kidney. Two other students in Seneviratne’s group, chemistry Ph.D. student Nimalee Jayasekera and junior biochemistry and molecular biology major Anderson Rivas, are already examining lipid metabolism in heart tissue. The results could eventually lead to reduced tissue damage in people taking drugs with known cardiac toxicity, such as some chemotherapy drugs.
Novel approach, new perspectives
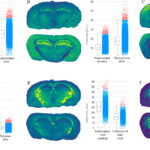
 Pharmacology & Translational Science featured the study on its cover. The two images on the right show the results from mass spectrometry performed on the mouse brain samples. The different colors represent the abundance of a particular lipid at each precise location.
Pharmacology & Translational Science featured the study on its cover. The two images on the right show the results from mass spectrometry performed on the mouse brain samples. The different colors represent the abundance of a particular lipid at each precise location.
The approach employed in the new study improves on traditional methods. Previously, researchers were forced to grind up tissue samples into a homogeneous slurry before analyzing them. The mass spectrometry method Phulara and Seneviratne used to image the samples maintains their integrity, and therefore retains spatial information. The results indicate which lipids are present, their abundance, and precisely where each lipid is located in a heat map for each tissue section.
Comparing the results of these studies between mice who had and had not been treated with Efavirenz showed that the drug altered the abundance of multiple lipids, particularly in the hippocampus, thalamus, and corpus callosum regions.
Then, when the researchers investigated the proteins present in the samples (their “proteome”), they found that 12 enzymes were much less abundant in treated mice. That list includes proteins involved in energy metabolism and lipid production and metabolism. The enzymes associated with the lipid changes will guide further investigation into the molecular mechanisms behind the changes.
“Combining tissue imaging with proteomics is extremely powerful, and that is one of the novel aspects of this work,” Seneviratne says. Top-of-the-line instrumentation available in Seneviratne’s lab and UMBC’s Interdisciplinary Life Sciences Building made the work possible, as well as collaboration with UMBC cancer biologists Charles Bieberich and Apurv Rege, who supported the drug treatment studies and are co-authors on the paper.
Digging deeper
 Herana Kamal Seneviratne (courtesy of Seneviratne)
Herana Kamal Seneviratne (courtesy of Seneviratne)
Now Phulara is homing in on how the enzymes identified in the current study affect lipid metabolism. To do that, he’ll manipulate the enzymes’ expression in different brain cell types and observe the effects. Some of the cells will receive Efavirenz and some will not. Observing the effects on lipid metabolism in the two groups will help reveal how the enzymes regulate lipid metabolism under normal conditions and how the drug disrupts that process.
Phulara has started by growing mouse astrocytes, a type of brain cell. He’ll collaborate with neurologists at the Johns Hopkins School of Medicine for analyses of human cells. Seneviratne’s group is also working on developing proteomic techniques that retain spatial information, similar to the lipid mass spectrometry imaging methods the team used.
“The overall goal is, ‘How can we target the lipid metabolism in order to minimize the side effects associated with this drug?’” Seneviratne says.
Earlier detection for better outcomes
For drugs that cause heart damage, such as the common chemotherapy drug doxorubicin, looking at lipid metabolism might help discover new mechanisms by which the damage occurs and help clinicians recognize the damage earlier. Typically, damage is not detected until it is quite advanced and irreversible, Seneviratne explains.
“We want to know the earliest molecular signatures of the damage. We think lipid metabolism will give us early molecular markers,” Seneviratne says. He adds, “The approaches that we developed in this study could be broadly applicable to kidney toxicities or cardio toxicities or any toxicities, and neurodegenerative diseases as well.”
“It’s really exciting. We can even find novel molecular targets for different diseases using this approach,” Phulara says. “If we see altered lipid metabolism in response to a specific drug, then we can target that altered lipid metabolism by supplementing with another drug, so hopefully that can help combat the disease.”





